Cold climates are a challenge to manufacturers with specialized epoxy coatings designed for immersion service. If coatings contractors don’t have sub-freezing temperatures or high humidity to contend with, then they probably have issues of low volatile organic compound (VOC) emissions, regulatory compliance, and user friendliness of the coating.
A conspicuous exception to the wisdom of abrasive blasting and coating application under shop conditions is the specter of waterblasting and field overcoating a failing coal tar epoxy with a surface tolerant epoxy. What would be even worse than that? The fact that on this project, it was the dead of winter, there was only a seven-day shut-down to complete the lining installation, and this was for the immersion of steel rakes in the primary clarifier of a pulp mill. Was the client expecting the impossible?
Surface Preparation and Coating Application
The client specified the application of a two-coat system of a modified phenalkamine epoxy to an Eimco rake and drive assembly of a 200-foot-diameter (61.0 m) primary clarifier. Even though the service temperature was ambient and the pH regularly buffered to be neutral, the existing coal tar epoxy had failed after several years of service.
As a solution, the mill engineer decided to apply the low temperature cure epoxy system in situ to the two long rake arms, center well, and launder ring. Each coat of epoxy was applied at approximately 6 mils (152.4 microns) dry film thickness (DFT) using 45:1 airless spray equipment.
Constrained by economic and environmental considerations, the method of surface preparation chosen by the mill engineer was waterblasting at 6,000–7,000 psi (41.4–48.3 MPa), followed by spot abrasive blasting of bare and damaged areas to meet NACE No. 2/Society for Protective Coatings (SSPC) Surface Preparation (SP) 10: Near-White Metal Blasting. They wanted to obtain a jagged 1.5- to 2.5-mil (38.1–63.5 microns) profile. Approximately 25 percent of the rakes were abrasive blasted in this way.
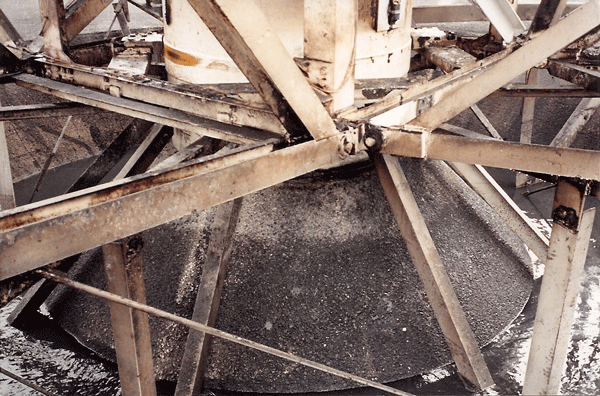
The contractors completed the work on schedule. Weather conditions were exceptionally grueling with temperatures dropping to freezing at night, and slush and rain showers occurred by day along with the occasional snow flurry. Before applying the first coat of epoxy coating, a gale force wind arose and tore up most of the tarping that the crew had applied for protection. This necessitated using rags and compressed air to quickly get rid of most of the water, but the substrate was still cold and damp when the coating was applied. According to the contractor at the time, “These were the worst conditions I have seen to apply any type of coating system.”
Interestingly, at the same time the contractors waterblasted the existing coal tar epoxy on the long rakes in situ, the mill painters completely sandblasted the two short arms of the rake assembly in the paint shop. Again, the steel was prepared to NACE No. 2/SSPC-SP-10 and the same two coats of phenalkamine epoxy were applied at approximately 6 mils (152.4 microns) DFT.
It was presumed by all present that the shop-applied and -cured coatings would outperform the field-applied coatings. But did they?
Coating Evaluation
After 3½ years in service, the primary clarifier was shut down in preparation for its new role as a secondary clarifier and for the installation of a Walker rake mechanism. What lessons would be learned from the coating application from hell?
First, overshadowing everything else, the phenalkamine epoxy coating was 99+ percent intact after more than three years of immersion over water-blasted coal tar epoxy. There was no evidence of any blistering. Ordinarily, coal tar epoxies must be roughened in order to obtain good adhesion for refreshment epoxy systems, even for dry conditions. Second, there was no difference in performance observed between two coats of the phenalkamine epoxy shop-applied over abrasive blasted steel (on the small rakes) and two coats of the phenalkamine epoxy field-applied over waterblasted coal tar epoxy steel (on the long rakes). Third, the field-applied phenalkamine epoxy system was easy to apply and demonstrated excellent immersion resistance even when having been applied in cold and damp winter conditions. Fourth, in a typical tank application in cold weather, the price at the time to heat the substrate can be 20 percent of the project — not including the scaffolding costs. No heat was used there. Fifth, the contractors and the painters had done an admirable job in challenging conditions.
It Worked?
So why does the unique modified phenalkamine technology work so well for marginally prepared surfaces in cold weather applications followed by water immersion?
The answer lies in the unique curing agent for this epoxy and the generation of the maximum concentration of adhesive sites generated (hydroxyl) that attach to cold surfaces even at temperatures as low as 0° F (-18° C). Winter grade curing agents and accelerators are not used because of the deleterious effects on coating performance. The built-in accelerator, or catalyst, actually comprises part of the structure of the converter itself. This intrinsically accelerates the reaction between the amine and epoxy groups even at low temperature 0° F (-18° C) without the applicator having to add an accelerator.
For the clarifier coating work, utilizing the modified phenalkamine technology enabled the contractors to continue working largely unimpeded by dew point concerns. Had a traditional epoxy been used it might have suffered “amine blush” or “sweat” at high humidity and low temperature. If not carefully removed by washing or cleaning with a suggested 1:1 glycol ether water mixture, this clear and water-soluble blush, or surface contaminant film, can lead to intercoat adhesion failure of a topcoat.
As evidenced in this project, amine blushing is not a problem for the modified phenalkamine epoxy technology. The reason: Rapid-cure epoxies of this type have some key attributes that discourage the amine blush phenomenon because of the curing agent, which contains mostly secondary amines. Since secondary amines do not react with CO2 and residual primary amines have been salted in an intra-molecular acid-base reaction, an amine blush is not produced. Even better, the molecular structure is very hydrophobic in the rapid cure modified phenalkamine epoxies due to the special curing agent technology. This discourages uptake of both moisture and polar gases, such as CO2, which lowers the concentration of gas in the film and further reduces any tendency to amine blush.
The problematic issue of “wet” versus “damp” surfaces was also addressed in this clarifier application. Again, the modified phenalkamine technology offered great advantages, which helped to account for the success of the project.
• These specialty epoxies were essentially neither moisture nor temperature sensitive.
• Common sense prevailed with respect to damp or wet surfaces. These epoxies were not (and should not be) applied to surfaces on which beaded or freestanding water was present.
• All substrates were (and must be) free of ice and frost.
Final Analysis
There are no miracle coatings, and contractors clearly wish to have the best possible conclusions for coating application. The success of this clarifier application boiled down to three major and overlapping components:
1. the unique coating chemistry suited to the application conditions and services,
2. proven testing and track records for similar requirements, and
3. good application of the coatings.
All things being equal, when three necessary requirements are successful and overlap, then as seen here, excellent performance can result — even during less-than-great conditions.
About the Authors
Dr. Mike O’Donoghue is the director of Engineering and Technical Services for AkzoNobel Coatings Ltd. (Canada). He has a Ph.D. in Inorganic Chemistry from the University of Surrey, England.
Vijay Datta is currently director of Protective Coatings, North America at International Paint LLC. He has a B.S. in Chemical Engineering from IIT Delhi, India, and an M.S. in Chemical Engineering from New Jersey Institute of Technology, N.J.
For more information, contact: AkzoNobel, www.akzonobel.com
